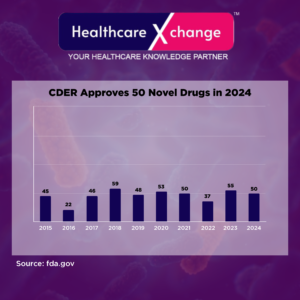
CDER Approves 50 Novel Drugs in 2024
- Data at Glance
- January 27, 2025
In 2024, the FDA’s Center for Drug Evaluation and Research (CDER) continued to drive medical innovation by approving 50 novel drugs, offering new treatment options for a wide range of diseases. These approvals, granted under New Drug Applications (NDAs) and Biologics License Applications (BLAs), introduce active ingredients that were previously unavailable in the U.S. healthcare landscape.
This milestone reflects CDER’s commitment to enhancing patient outcomes, addressing unmet medical needs, and fostering pharmaceutical advancements. Below is a breakdown of these approvals, highlighting key trends and therapeutic breakthroughs shaping the future of medicine.
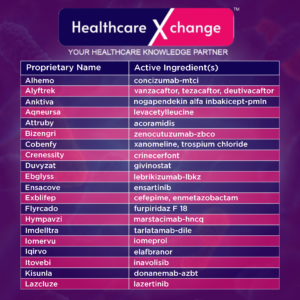
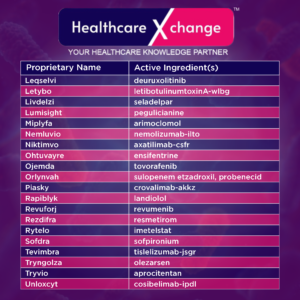
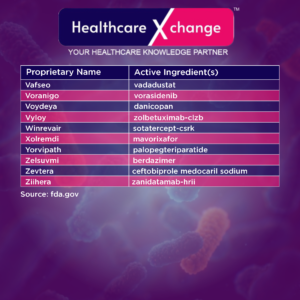
Note: *The information is current as of December 31, 2024. In rare cases, CDER may change a drug’s NME designation or status as a new BLA.
From 2015 to 2024, the CDER averaged 47 novel drug approvals per year, as shown in a 10-year graph.